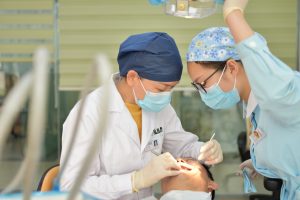
The Scottish Dental Association welcomed the decision of the Chief Dental Officer to allow dental practices to see their own patients for urgent care involving AGPs from the 17th of August. However, having noted that the 3M masks issued to some practices had original “use by” dates going back to 2012, we have had serious concerns about the decision to issue these ostensibly revalidated masks.
We drafted a letter sharing our concerns with the intention of forwarding this to the relevant members of the Scottish Government. Having researched the history and timeline of the revalidation process and the decision to issue the masks, the letter was produced.
Two unexpected things happened which gave us reason to pause and reconsider the forwarding of this missive. Yesterday, the BDA issued guidance advising against use of the masks, and today, the manufacturer, 3M, appear to have openly stated that the masks should not be used.
In view of the above, it would appear that the use of the masks surely must cease forthwith, and thus, our letter which strove to achieve the same is now redundant. As such, and to allow the Scottish Government and CDO to focus on a satisfactory resolution to this crisis, we have taken the decision not to engage with them on this matter. We do, though, remain fully committed to our stated aim of helping in any way possible, to increase the quality of patient care available.
We also feel that much of the content of the letter is of interest to all members the profession, and since not everyone will be fully aware of the facts surrounding this issue, we have decided to share the content as an open letter.
On Friday 28th August 2020 the British Dental Association advised members not to use 3M FFP3 masks supplied by NSS, for the purpose of use during Aerosol Generating Procedures (AGPs) in general dental practice. This conflicts with NSS advice which assured us that these masks, with initial use by dates from 2012, have been revalidated and are fit for purpose. Indeed, many dentists have confirmed usage of these masks having issued them to their staff in recent weeks.
In view of this, and having had sight of the revalidation report as issued by Inspec to the NSS in March this year, the Scottish Dental Association have grave concerns about the use of these masks. The Chief Medical Officer and Chief Nursing Officer gave assurances that “stock has passed a series of stringent quality assurance tests. NHS National Services Scotland (NSS) this week decided to release “the certification confirming these are safe and fit for purpose.” Having viewed these documents and considered the implications of FFP3 masks intended for aerosol generating procedures (AGPs), we disagree with the CMO/CNO/NSS assurances on safety.
The tests which were carried out seem not to be “stringent”, nor were they “passed” by Inspec as per their definition of “passed.” They have provided limited and inconclusive results, given that only 3 of the 17 ‘clauses’ or aspects of testing were requested by NHS Supply Chain. Those which were tested elicited ‘Ltd’ results, meaning that “testing required was insufficient completely to verify compliance with the clause.” This was the case for every clause tested on every batch although only one mask from each batch was tested. Furthermore, Inspec state that “the effect on materials for ‘in use’ environmental factors could not be evaluated during laboratory tests. Manufacturer to testify regarding such factors.”
3M have stated that they did not testify regarding these masks as suggested by Inspec. Please confirm the reasoning behind the decision not to involve the manufacturer.
Neither ‘Practical Performance’ nor ‘Total inward leakage’ was tested and none of the clauses tested elicited a ‘Pass’ (requirement satisfied) according to the Inspec document, for any mask tested. Please explain the reasoning behind the limited testing requested and the ambiguity which exists between their term “Ltd” and the term “Passed” as used by the the CMO/CNO.
Given that dental practices were instructed to remain closed through the ‘lockdown’ period by the Chief Dental Officer in the week following the FFP3 respirator testing detailed above, it was assumed that the decision to enforce closures was based on the limited supplies of PPE. Did the CDO take a view on the suitability of these masks for dental AGPs at that juncture?
We feel that, in view of the large stockpile of pre-existing PPE, insufficient consideration was given to the early roll out of a fit testing programme which might have allowed a more comprehensive provision of urgent dental care in the early stages of the pandemic. We reflect on the fact that the inability to offer essential dental care, due to the apparent lack of available PPE, contributed greatly to the suffering and lasting damage to our patient’s dental health.
We have a duty of care to our staff and patients and do wonder, in the event of an adverse occurrence related to the use of these masks, whether liability falls upon the NSS, local Health Board authority, practice employers and public liability insurers.
We seek confirmation in regard to an improved and expedited supply of FFP3 masks in order that staff and patients might be confident of a safe return to our practices. We look forward to an urgent response to our questions above and welcome a meeting at your earliest convenience to discuss our concerns.
Interim Committee
The Scottish Dental Association
Additional Reading
Department of Health – Test Certification, 19 March 2020
CNO/CMO – Statement on Revalidated PPE, 26 March 2020
The BDA – Scotland Dentists Urged to Drop Out of Date PPE, 28 August 2020
The Times – You shouldn’t use out of date masks says manufacturer, 29 August 2020